Addition and Removal of Mercury in Jiulong River Estuary during Mixing of Seawater and Freshwater
-
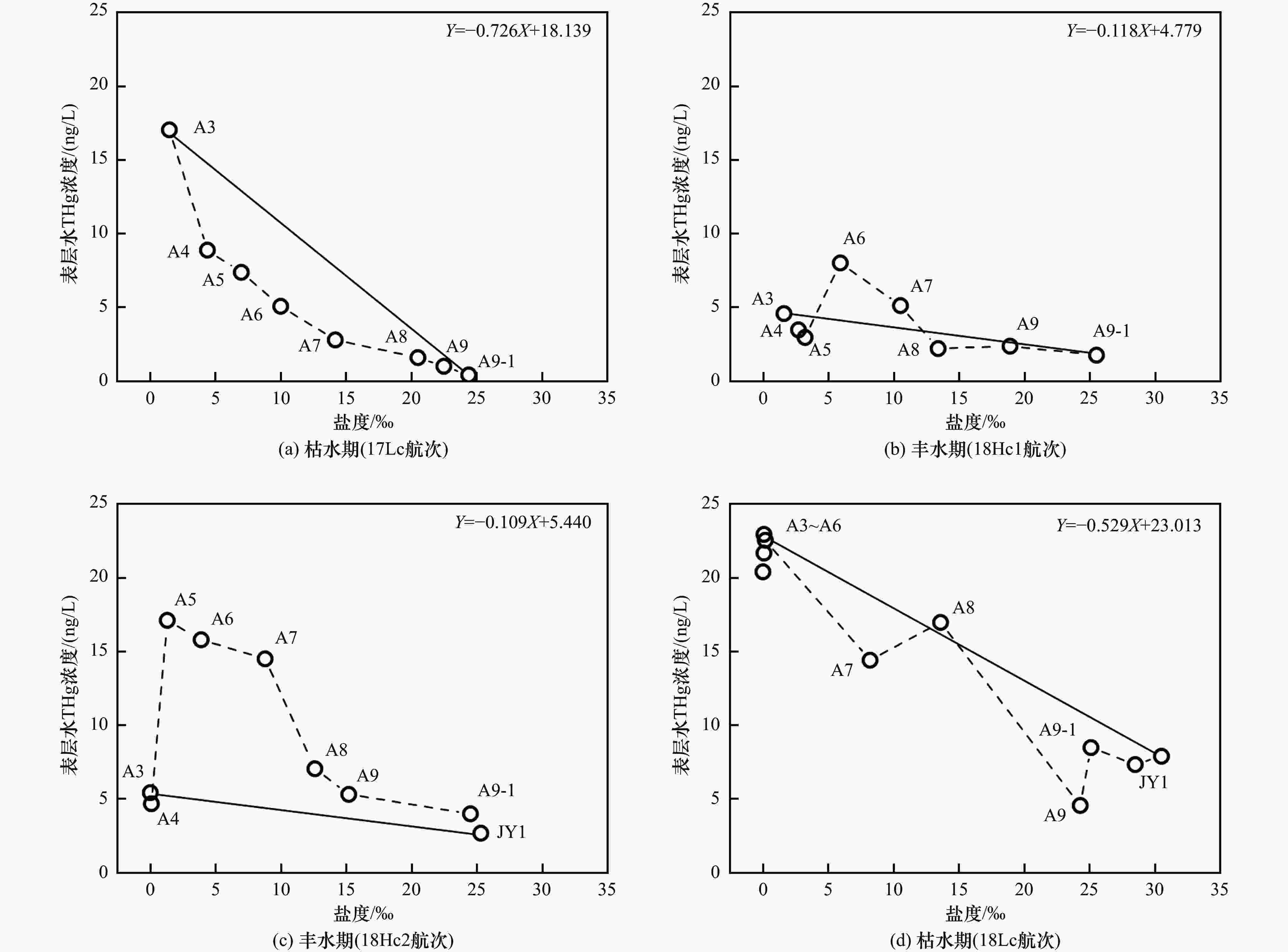
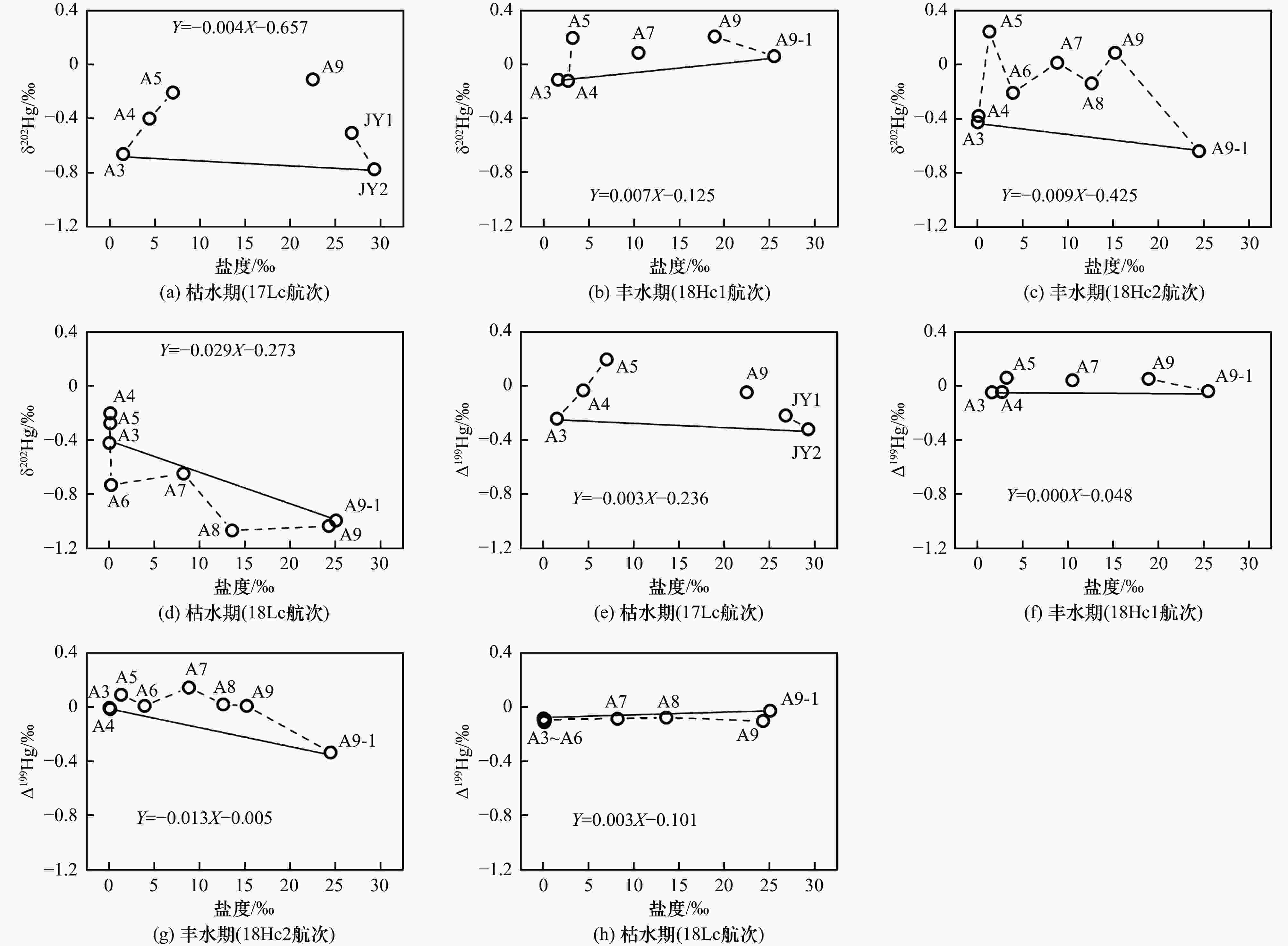
摘要: 河口是海陆界面能量和物质交换的重要场所,研究汞在河口的迁移特征及影响因素,对认识汞的生物地球化学行为具有重要意义. 本研究测定了九龙江河口区沉积物孔隙水的总汞(total mercury, THg)浓度与汞同位素特征,结合笔者所在课题组已发表的相关研究数据,比较不同季节、不同潮位、降水与否的表层水THg浓度、汞同位素组成与盐度之间的关系,探究影响表层水中汞额外增加/消除的因素及机制. 结果表明:①孔隙水THg浓度〔(38.28±28.80) ng/L,n=28〕显著高于表层水〔(8.53±6.85) ng/L,n=35〕(P<0.01);孔隙水THg浓度受季节或径流量变化的影响不明显. 孔隙水δ202Hg平均值(−1.24‰±0.36‰,n=28)处于表层水(−0.32‰±0.38‰,n=29)和沉积物(−1.99‰±0.69‰,n=18)之间;Δ199Hg平均值(−0.13‰±0.03‰,n=28)低于表层水(−0.04‰±0.10‰,n=29)和沉积物(−0.04‰±0.16‰,n=18),表明汞在孔隙水与沉积物间的双向传递与吸附及非光致氧化过程有关. ②枯水期河口区表层水中的汞以额外消除为主,呈现汞“汇”特征;丰水期则呈现汞“源”特征. 当咸淡水在河道较浅处交汇时,表层水中汞的额外增加更大. 无论是增加或消除,表层水中汞的δ202Hg与Δ199Hg值均升高. 汞在咸淡水交汇界面的迁移受径流量、潮位、河道特征及降水事件等多因素影响. ③枯水期中降水事件发生时表层水THg浓度和汞同位素组成随盐度的变化特征与非降水期不同,表明降水可影响表层水中汞的行为. 研究显示,汞在咸淡水交汇界面的迁移受径流量、潮位、河道特征及降水事件等多因素影响,河口是汞“源”也是汞“汇”.Abstract: Estuaries play an essential role in the exchange of energy and materials at the sea-land interface. Studying the migration characteristics of mercury (Hg) and related influencing factors is of great significance for understanding the biogeochemical cycling of Hg. This study assessed total Hg (THg) concentration and Hg isotope compositions in sediment porewater of the Jiulong River Estuary (JRE). Combined with data from relevant published studies published by author′s research group, the relationship between THg concentration, Hg isotopic composition, and surface water salinity in different seasons (wet and dry seasons), different tidal levels, and with or without precipitation events were compared to determine the factors and mechanisms affecting the addition/removal patterns of Hg in surface water. The results showed that: (1) The average porewater THg concentration ((38.28±28.80) ng/L, n=28) was significantly higher than that in surface water ((8.53±6.85) ng/L, n=35) (P<0.01); Porewater THg concentration was not significantly affected by seasons and runoff. On the other hand, the average δ202Hg value of porewater (−1.24‰±0.36‰, n=28) was between that of surface water (−0.32‰±0.38‰, n=29) and sediment (−1.99‰±0.69‰, n=18), while the average Δ199Hg value (−0.13‰±0.03‰, n=28) of porewater was lower than that of surface water (−0.04‰±0.10‰, n=29) and sediment (−0.04‰±0.16‰, n=18), indicating that the bidirectional Hg transfer between porewater and sediment was related to the adsorption and abiotic dark oxidation processes. (2) Hg in the surface water of JRE was mainly removed during the dry season, acting as a Hg sink, while during the wet season, the river estuary was the source of Hg. In addition, significant increases in Hg addition in the surface water were observed when freshwater and seawater converged in the shallow channel. Regardless of the addition/removal pattern of Hg, the δ202Hg and Δ199Hg values increased among almost all samples. The Hg migration at the converge interface of freshwater and seawater was affected by several factors, such as runoff, tide level, channel characteristics, and precipitation events. (3) The variation characteristics of THg concentration and Hg isotopic composition with salinity in surface water during the precipitation period were different from those in the non-precipitation period, indicating the effects of precipitation events on the Hg behavior in surface water during the dry season. It is concluded that Hg migration at the converge interface of freshwater and seawater was affected by several factors, such as runoff, tide level, channel characteristics, and precipitation events. In addition, the river estuary was both source and sink of Hg.
-
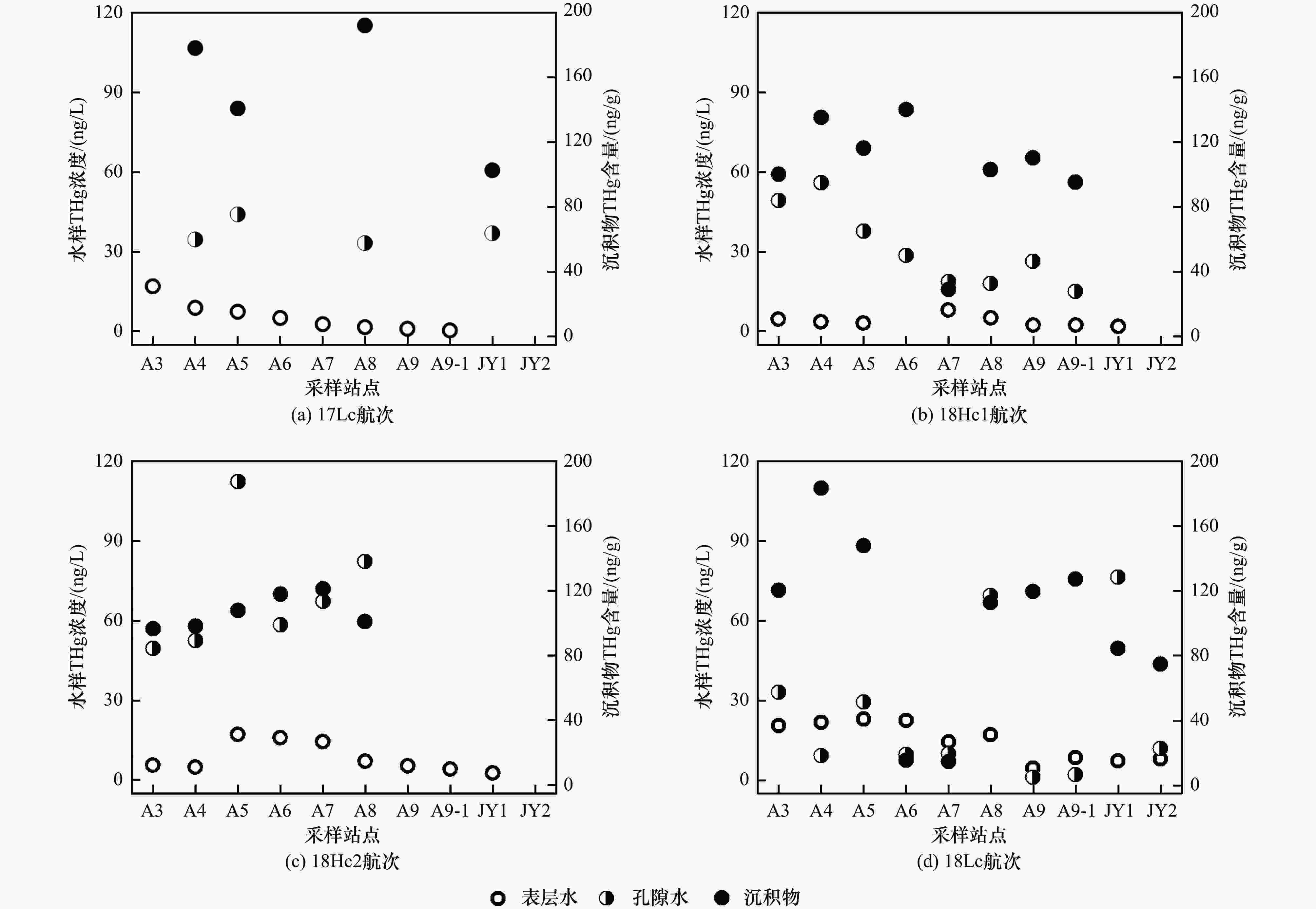
图 2 九龙江河口各航次表层水、孔隙水THg浓度及沉积物THg含量分布
注:表层水THg浓度与沉积物THg含量数据均源自笔者所在课题组前期研究结果[32].
Figure 2. Distribution of THg concentrations in surface water, porewater, and sediment samples collected from Jiulong River Estuary (JRE)
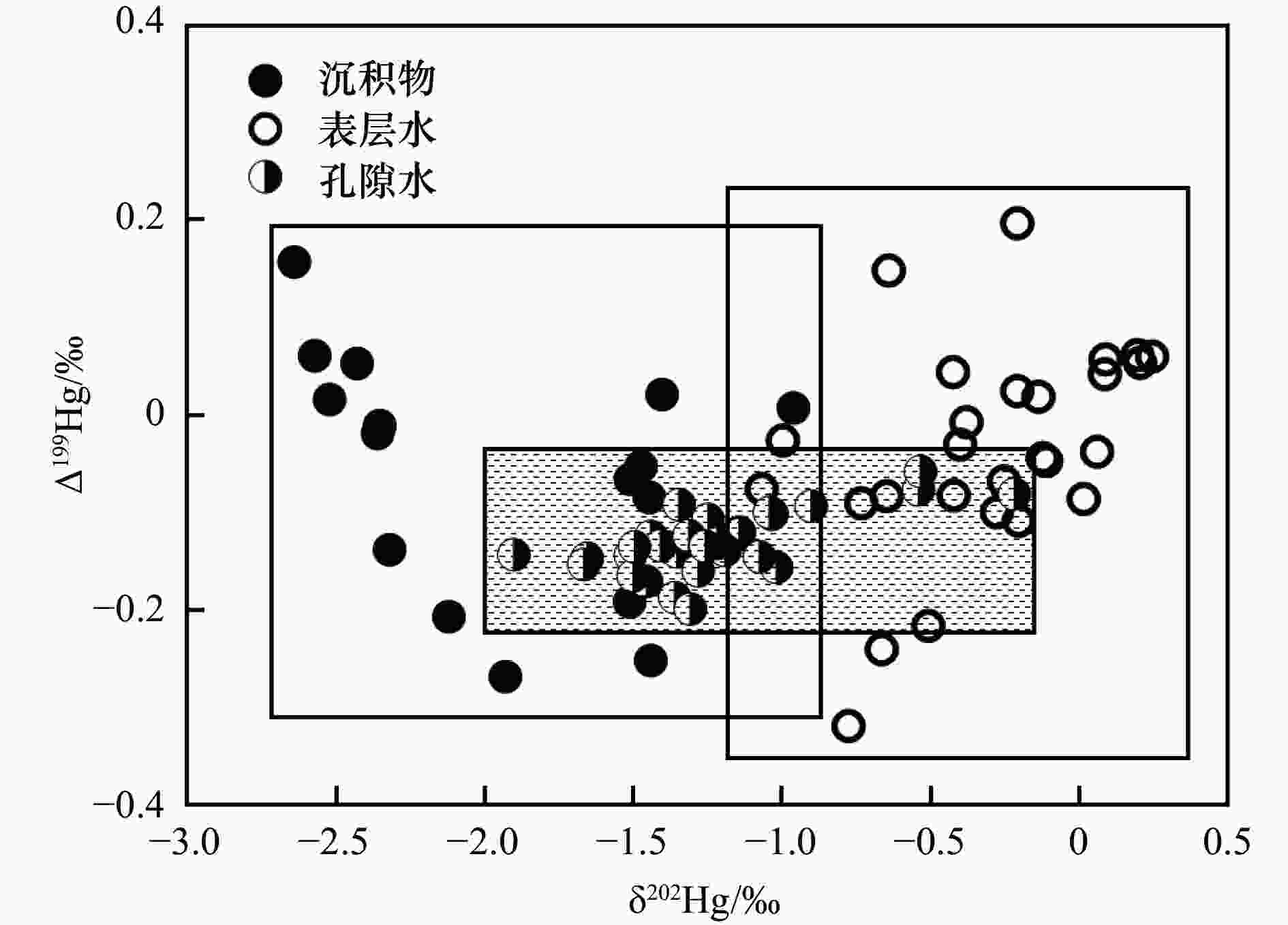
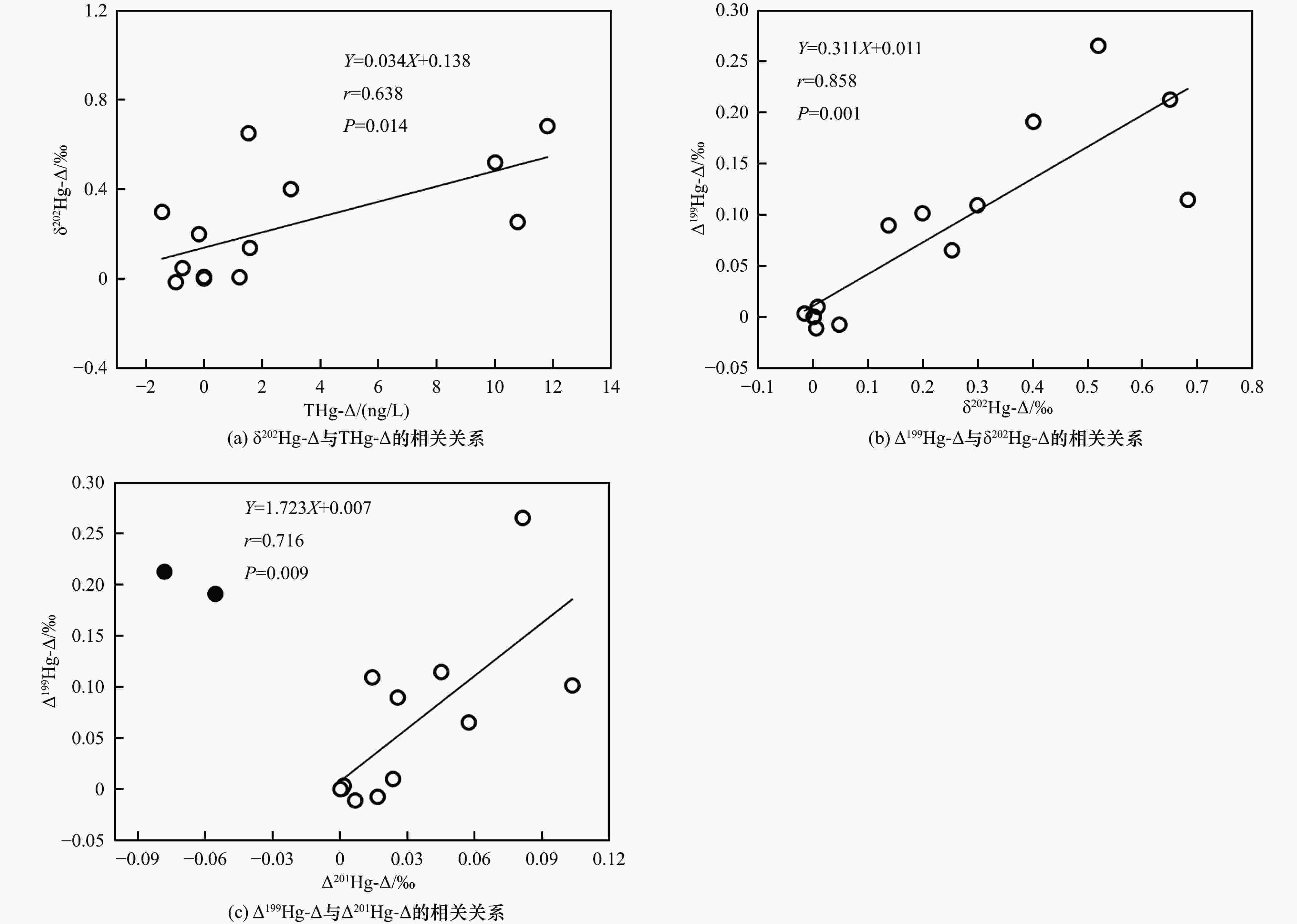
图 3 九龙江河口区表层水、孔隙水及沉积物的Δ199Hg与δ202Hg关系
注:表层水与沉积物汞同位素δ202Hg、Δ199Hg的数据均源自笔者所在课题组前期研究结果[32];方框代表沉积物或表层水样品坐标点的分布范围;阴影代表孔隙水样品坐标点分布范围.
Figure 3. Relationships between Δ199Hg and δ202Hg in surface water, porewater, and sediment samples of JRE
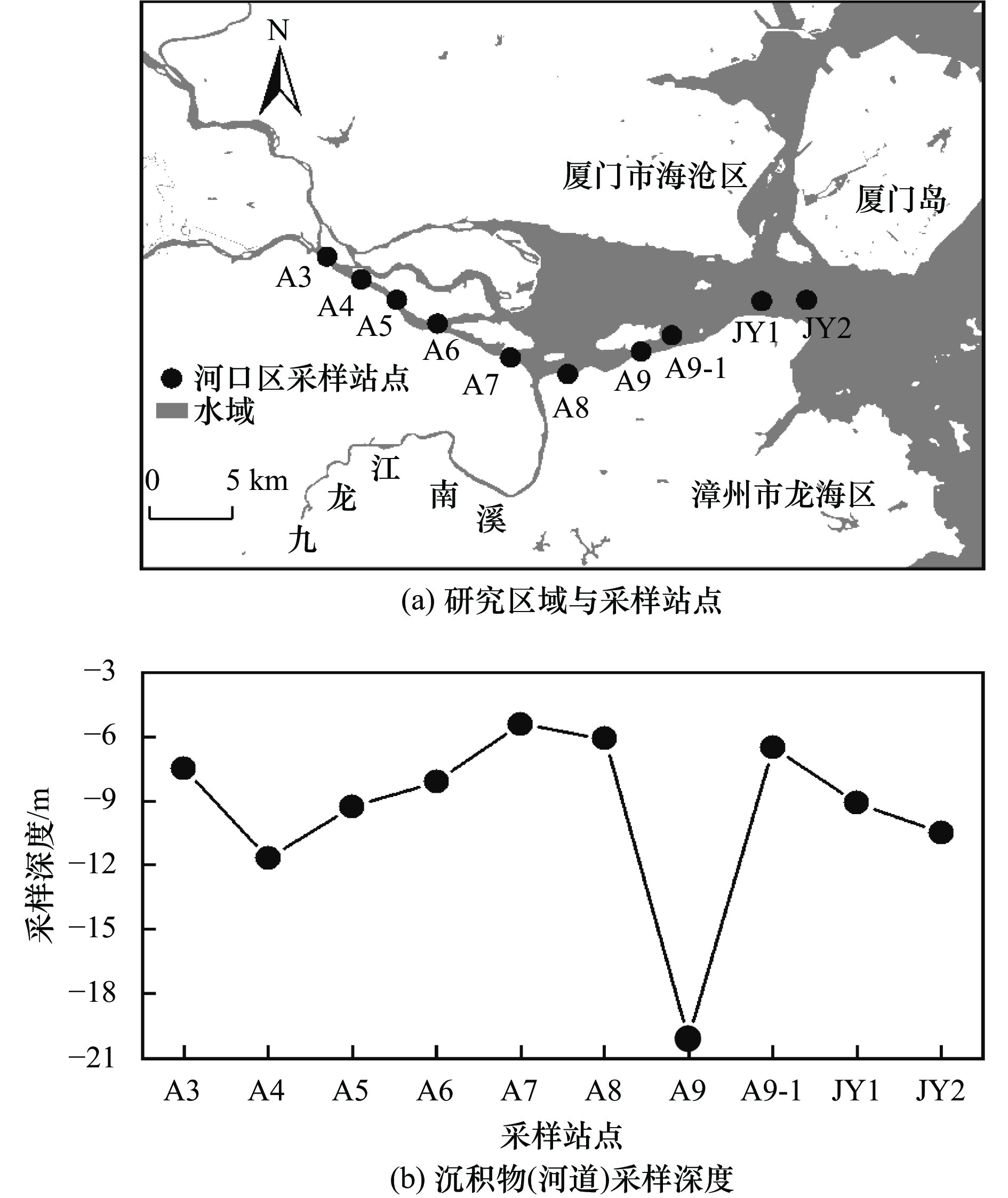
表 1 九龙江河口区孔隙水THg浓度、汞同位素组成及表层水盐度
Table 1. THg concentrations and isotopic compositions of JRE porewater samples, and salinity of JRE surface water samples
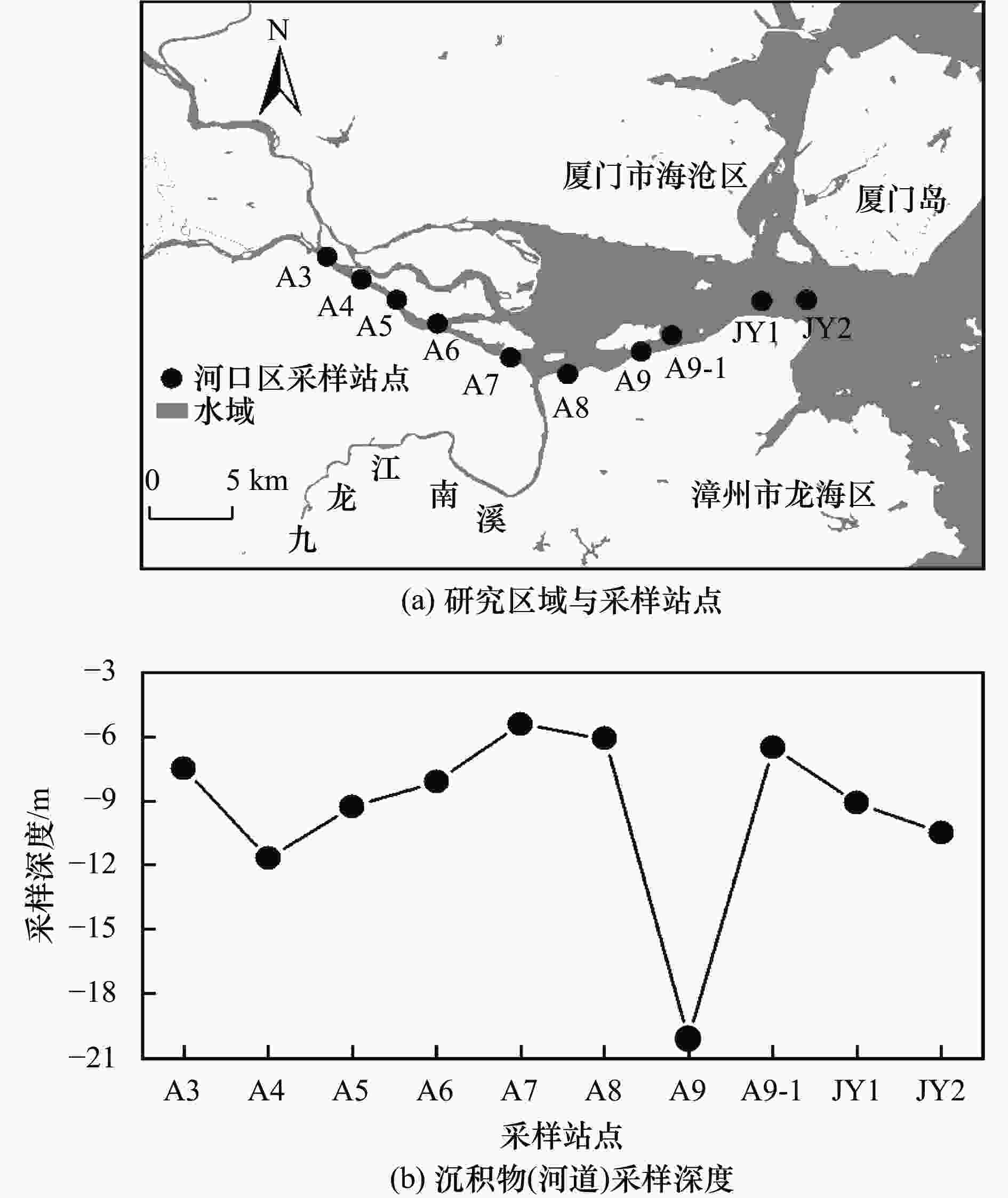
航次 站点名称 THg浓度/(ng/L) 汞同位素组成 表层水盐度/‰ δ202Hg/‰ Δ199Hg/‰ Δ201Hg/‰ 17Lc(枯水期) A3 — — — — 1.5 A4 34.66 −1.37 −0.14 −0.15 4.4 A5 44.15 −1.35 −0.09 −0.14 7.0 A6 — — — — 10.0 A7 — — — — 14.2 A8 33.28 −1.65 −0.15 −0.18 20.5 A9 — — — — 22.5 A9-1 — — — — 24.4 JY1 36.95 −0.54 −0.08 −0.02 26.8 JY2 — — — — 29.3 18Hc1(丰水期) A3 49.27 −1.41 −0.13 −0.17 1.6 A4 55.86 −1.66 −0.15 −0.16 2.7 A5 37.51 −1.50 −0.17 −0.21 3.2 A6 28.44 −1.36 −0.19 −0.21 5.9 A7 18.7 −1.50 −0.14 −0.16 10.5 A8 17.91 −1.19 −0.14 −0.14 13.4 A9 26.33 −1.31 −0.12 −0.15 18.9 A9-1 15.1 −1.31 −0.20 −0.18 25.5 JY1 — — — — — JY2 — — — — — 18Hc2(丰水期) A3 49.48 −1.90 −0.14 −0.26 0.0 A4 52.39 −0.90 −0.09 −0.13 0.1 A5 112.20 −1.04 −0.10 −0.18 1.3 A6 58.27 −1.22 −0.13 −0.13 3.9 A7 67.06 −1.26 −0.13 −0.14 8.8 A8 82.17 −1.07 −0.15 −0.15 12.6 A9 — — — — 15.2 A9-1 — — — — 24.5 JY1 — — — — 25.3 JY2 — — — — — 18Lc(枯水期,强降雨) A3 33.12 −1.51 −0.14 0.02 0.0 A4 9.25 −1.25 −0.11 −0.01 0.1 A5 29.38 −1.35 −0.14 0.00 0.1 A6 9.70 −0.22 −0.08 0.04 0.2 A7 9.96 −0.53 −0.06 0.07 8.2 A8 69.32 −1.44 −0.12 0.03 13.6 A9 1.09 −1.28 −0.16 −0.01 24.3 A9-1 2.13 −1.14 −0.12 −0.01 25.1 JY1 76.27 −1.45 −0.17 0.02 30.5 JY2 11.81 −1.02 −0.16 0.05 28.5 注:—代表未检出. -
[1] 王橹玺,李慧,张文杰,等.大气PM2.5载带重金属的区域污染特征研究[J].环境科学研究,2021,34(4):849-862.WANG L X,LI H,ZHANG W J,et al.Study on the regional pollution characteristics of heavy metals in PM2.5[J].Research of Environmental Sciences,2021,34(4):849-862. [2] 徐源,师华定,王超,等.湖南省郴州市苏仙区重点污染企业影响区的土壤重金属污染源解析[J].环境科学研究,2021,34(5):1213-1222. doi: 10.13198/j.issn.1001-6929.2020.11.03XU Y,SHI H D,WANG C,et al.Heavy metal pollution sources in soil affected by key pollution enterprises in Suxian District,Chenzhou City,Hunan Province[J].Research of Environmental Sciences,2021,34(5):1213-1222. doi: 10.13198/j.issn.1001-6929.2020.11.03 [3] 旷攀,李秋华,金爽,等.贵州高原普定水库水环境重金属的时空分布特征及风险评价[J].环境科学研究,2021,34(3):576-588.KUANG P,LI Q H,JIN S,et al.Spatial and temporal distribution of heavy metals in water enviroment of Puding Reservoir in Guizhou Province and risk assessment[J].Research of Environmental Sciences,2021,34(3):576-588. [4] REINFELDER J R,JANSSEN S E.Tracking legacy mercury in the Hackensack River estuary using mercury stable isotopes[J].Journal of Hazardous Materials,2019,375:121-129. doi: 10.1016/j.jhazmat.2019.04.074 [5] 王立军,刘亮,马新东,等.大连湾表层沉积物中总汞和甲基汞的分布及影响因素[J].环境科学研究,2019,32(6):1026-1032.WANG L J,LIU L,MA X D,et al.Total mercury and methylmercury distributions in surface sediment in Dalian Bay and the factors driving the distributions[J].Research of Environmental Sciences,2019,32(6):1026-1032. [6] 李嗣新.蓄水对水库下游食物网中汞生物累积影响研究进展[J].环境科学研究,2018,31(8):1346-1356. doi: 10.13198/j.issn.1001-6929.2018.04.07LI S X.A review on the studies related to the impact of impoundment on mercury bioaccumulation in food web downstream from reservoir[J].Research of Environmental Sciences,2018,31(8):1346-1356. doi: 10.13198/j.issn.1001-6929.2018.04.07 [7] KWON S Y,BLUM J D,NADELHOFFER K J,et al.Isotopic study of mercury sources and transfer between a freshwater lake and adjacent forest food web[J].Science of the Total Environment,2015,532:220-229. doi: 10.1016/j.scitotenv.2015.06.012 [8] 孔林,刘杰民,韦艳,等.贵州省典型铅锌矿区居民血液总汞和甲基汞暴露及健康风险模型预测评估[J].环境科学研究,2021,34(6):1499-1508. doi: 10.13198/j.issn.1001-6929.2021.03.08KONG L,LIU J M,WEI Y,et al.Total mercury and methyl mercury in blood of inhabitant and their associated modelling prediction evaluation in typical lead-zinc mining region,Guizhou Province,China[J].Research of Environmental Sciences,2021,34(6):1499-1508. doi: 10.13198/j.issn.1001-6929.2021.03.08 [9] JIANG G B,SHI J B,FENG X B.Mercury pollution in China[J].Environmental Science & Technology,2006,40(12):3672-3678. [10] 陈能汪.全球变化下九龙江河流-河口系统营养盐循环过程、通量与效应[J].海洋地质与第四纪地质,2018,38(1):23-31. doi: 10.16562/j.cnki.0256-1492.2018.01.003CHEN N W.Nutrient cycling processes,fluxes and effects in the Jiulong River-estuary system under global change[J].Marine Geology & Quaternary Geology,2018,38(1):23-31. doi: 10.16562/j.cnki.0256-1492.2018.01.003 [11] YU D,CHEN N W,KROM M D,et al.Understanding how estuarine hydrology controls ammonium and other inorganic nitrogen concentrations and fluxes through the subtropical Jiulong River Estuary,S.E. China under baseflow and flood-affected conditions[J].Biogeochemistry,2019,142(3):443-466. doi: 10.1007/s10533-019-00546-9 [12] 郭民权,江毓武.九龙江河口洪水期悬沙及冲於变化的数值模拟[J].厦门大学学报(自然科学版),2010,49(5):688-693.GUO M Q,JIANG Y W.Distribution of suspended sediment and erosion simulation of the Jiulong River Estuary during a flood process[J].Journal of Xiamen University (Natural Science),2010,49(5):688-693. [13] CAO W Z,HUANG Z,ZHAI W D,et al.Isotopic evidence on multiple sources of nitrogen in the northern Jiulong River,southeast China[J].Estuarine,Coastal and Shelf Science,2015,163:37-43. doi: 10.1016/j.ecss.2015.05.042 [14] YIN X J,LIN Y P,LIANG C C,et al.Source and fate of dissolved inorganic carbon in Jiulong River,southeastern China[J].Estuarine,Coastal and Shelf Science,2020,246:107031. doi: 10.1016/j.ecss.2020.107031 [15] SANIEWSKA D,BEŁDOWSKA M,SZYMCZAK E,et al.Processes affecting the transformation of mercury in the coastal zone in the vicinity of two river mouths in the southern Baltic Sea[J].Marine Chemistry,2022,238:104065. doi: 10.1016/j.marchem.2021.104065 [16] BOYLE E,COLLIER R,DENGLER A T,et al.On the chemical mass-balance in estuaries[J].Geochimica et Cosmochimica Acta,1974,38(11):1719-1728. doi: 10.1016/0016-7037(74)90188-4 [17] OFFICER C B.Discussion of the behaviour of nonconservative dissolved constituents in estuaries[J].Estuarine and Coastal Marine Science,1979,9(1):91-94. doi: 10.1016/0302-3524(79)90009-4 [18] LIU M D,ZHANG Q R,MAAVARA T,et al.Rivers as the largest source of mercury to coastal oceans worldwide[J].Nature Geoscience,2021,14(9):672-677. doi: 10.1038/s41561-021-00793-2 [19] CROWTHER E R,DEMERS J D,BLUM J D,et al.Use of sequential extraction and mercury stable isotope analysis to assess remobilization of sediment-bound legacy mercury[J].Environmental Science Processes & Impacts,2021,23(5):756-775. [20] WANG T,OBRIST D.Inorganic and methylated mercury dynamics in estuarine water of a salt marsh in Massachusetts,USA[J].Environmental Pollution,2022,294:118657. doi: 10.1016/j.envpol.2021.118657 [21] 魏珈,郭卫东,王志恒,等.降雨事件对不同流域背景河流DOM组成及入海通量的影响[J].农业环境科学学报,2016,35(4):737-744. doi: 10.11654/jaes.2016.04.018WEI J,GUO W D,WANG Z H,et al.Impacts of storm event on DOM composition and flux in two Jiulong Tributaries with different watershed features[J].Journal of Agro-Environment Science,2016,35(4):737-744. doi: 10.11654/jaes.2016.04.018 [22] 冯新斌,尹润生,俞奔,等.汞同位素地球化学概述[J].地学前缘,2015,22(5):124-135. doi: 10.13745/j.esf.2015.05.010FENG X B,YIN R S,YU B,et al.A review of Hg isotope geochemistry[J].Earth Science Frontiers,2015,22(5):124-135. doi: 10.13745/j.esf.2015.05.010 [23] BLUM J D,SHERMAN L S,JOHNSON M W.Mercury isotopes in earth and environmental sciences[J].Annual Review of Earth and Planetary Sciences,2014,42:249-269. doi: 10.1146/annurev-earth-050212-124107 [24] WANG X,WU X Y,CHEN M,et al.Isotopic constraint on the sources and biogeochemical cycling of nitrate in the Jiulong River Estuary[J].Journal of Geophysical Research:Biogeosciences,2021,126(3):e2020JG005850. [25] GUO W D,STEDMON C A,HAN Y C,et al.The conservative and non-conservative behavior of chromophoric dissolved organic matter in Chinese estuarine waters[J].Marine Chemistry,2007,107(3):357-366. doi: 10.1016/j.marchem.2007.03.006 [26] US Environmental Protection Agency.Method 1631:mercury in water by oxidation,purge and trap,and cold vapor atomic fluorescence spectrometry[S].Washington DC:US Environmental Protection Agency,1996. [27] 梁英,袁东星,章臻.环境样品中总汞测定方法的建立:吹扫-金柱捕集-(双柱)热脱附-冷原子荧光法[J].光谱实验室,2010,27(6):2111-2117. doi: 10.3969/j.issn.1004-8138.2010.06.001LIANG Y,YUAN D X,ZHANG Z.Determination of total mercury in environmental samples by cold vapor atomic fluorescence spectrometry with purge-trap,dual-trap amalgamation[J].Chinese Journal of Spectroscopy Laboratory,2010,27(6):2111-2117. doi: 10.3969/j.issn.1004-8138.2010.06.001 [28] SUN L M,LU B Y,YUAN D X,et al.Variations in the isotopic composition of stable mercury isotopes in typical mangrove plants of the Jiulong Estuary,SE China[J].Environmental Science and Pollution Research,2017,24(2):1459-1468. doi: 10.1007/s11356-016-7933-1 [29] LIN H Y,YUAN D X,LU B Y,et al.Isotopic composition analysis of dissolved mercury in seawater with purge and trap preconcentration and a modified Hg introduction device for MC-ICP-MS[J].Journal of Analytical Atomic Spectrometry,2015,30(2):353-359. doi: 10.1039/C4JA00242C [30] BLUM J D,BERGQUIST B A.Reporting of variations in the natural isotopic composition of mercury[J].Analytical and Bioanalytical Chemistry,2007,388(2):353-359. doi: 10.1007/s00216-007-1236-9 [31] HUANG S Y,SUN L M,ZHOU T J,et al.Natural stable isotopic compositions of mercury in aerosols and wet precipitations around a coal-fired power plant in Xiamen,southeast China[J].Atmospheric Environment,2018,173:72-80. doi: 10.1016/j.atmosenv.2017.11.003 [32] ZHANG X D,SUN L M,HUANG X X,et al.Mercury sources and processes implied by other pollutants distributions in surface water and sediments of a subtropical estuary in southern China[J].Water,Air,& Soil Pollution,2022,233(8):1-19. [33] 钱晓莉,冯新斌,闭向阳,等.贵州省草海表层水体和沉积物间隙水中汞的含量和形态分布初步研究[J].湖泊科学,2008,20(5):563-570. doi: 10.3321/j.issn:1003-5427.2008.05.003QIAN X L,FENG X B,BI X Y,et al.Concentrations and distributions of mercury species in surface water and porewater of Lake Caohai,Guizhou Province[J].Journal of Lake Sciences,2008,20(5):563-570. doi: 10.3321/j.issn:1003-5427.2008.05.003 [34] JISKRA M,WIEDERHOLD J G,BOURDON B,et al.Solution speciation controls mercury isotope fractionation of Hg(Ⅱ) sorption to goethite[J].Environmental Science & Technology,2012,46(12):6654-6662. [35] ZHENG W,DEMERS J D,LU X,et al.Mercury stable isotope fractionation during abiotic dark oxidation in the presence of thiols and natural organic matter[J].Environmental Science & Technology,2019,53(4):1853-1862. [36] HUANG S Y,ZHAO Y H,LV S P,et al.Distribution of mercury isotope signatures in Yundang Lagoon,Xiamen,China,after long-term interventions[J].Chemosphere,2021,272:129716. doi: 10.1016/j.chemosphere.2021.129716 [37] ZHENG W,OBRIST D,WEIS D,et al.Mercury isotope compositions across North American forests[J].Global Biogeochemical Cycles,2016,30(10):1475-1492. doi: 10.1002/2015GB005323 [38] CHEN N W,KROM M D,WU Y Q,et al.Storm induced estuarine turbidity maxima and controls on nutrient fluxes across river-estuary-coast continuum[J].Science of the Total Environment,2018,628/629:1108-1120. doi: 10.1016/j.scitotenv.2018.02.060 [39] 刘楠涛,吴飞,袁巍,等.长江与黄河源丰水期地表水中汞的分布特征、赋存形态及来源解析[J].环境科学,2022,43(11):5064-5072. doi: 10.13227/j.hjkx.202201143LIU N T,WU F,YUAN W,et al.Mercury speciation,distribution,and potential sources in surface waters of the Yangtze and Yellow River source basins of Tibetan Plateau during wet season[J].Environmental Science,2022,43(11):5064-5072. doi: 10.13227/j.hjkx.202201143 [40] LIN H Y,PENG J J,YUAN D X,et al.Mercury isotope signatures of seawater discharged from a coal-fired power plant equipped with a seawater flue gas desulfurization system[J].Environmental Pollution,2016,214:822-830. doi: 10.1016/j.envpol.2016.04.059 [41] ZHENG W,HINTELMANN H.Nuclear field shift effect in isotope fractionation of mercury during abiotic reduction in the absence of light[J].The Journal of Physical Chemistry A,2010,114(12):4238-4245. doi: 10.1021/jp910353y [42] WIEDERHOLD J G,CRAMER C J,DANIEL K,et al.Equilibrium mercury isotope fractionation between dissolved Hg(Ⅱ) species and thiol-bound Hg[J].Environmental Science & Technology,2010,44(11):4191-4197. -




 下载:
下载: